CT's Rare Disease Report Card Reflects Good Grades, Not-so-Good Grades
/Thanks to innovative new treatments, diseases that were once fatal are now being treated as chronic conditions. But these breakthrough treatments will be out of reach for many patients, according to the National Organization for Rare Diseases, because health plans are using deductibles and coinsurance to shift more of the cost of medication onto the patients who rely on those treatments. The national organization, which is headquartered in Danbury and Washington, DC, explains that taken together, those out-of-pocket costs are outpacing wages, and patients are left struggling.
 To assist patients who find themselves in this difficult situation, several states have passed legislation mandating a limit on out-of-pocket costs for medications. These limits can be applied in different forms, such as a per-drug cap or by mandating a copay-only structure in certain health plans. Those are just some of the areas of particular interest to NORD, which advocates for patients – and their families – facing the challenges of rare diseases.
To assist patients who find themselves in this difficult situation, several states have passed legislation mandating a limit on out-of-pocket costs for medications. These limits can be applied in different forms, such as a per-drug cap or by mandating a copay-only structure in certain health plans. Those are just some of the areas of particular interest to NORD, which advocates for patients – and their families – facing the challenges of rare diseases.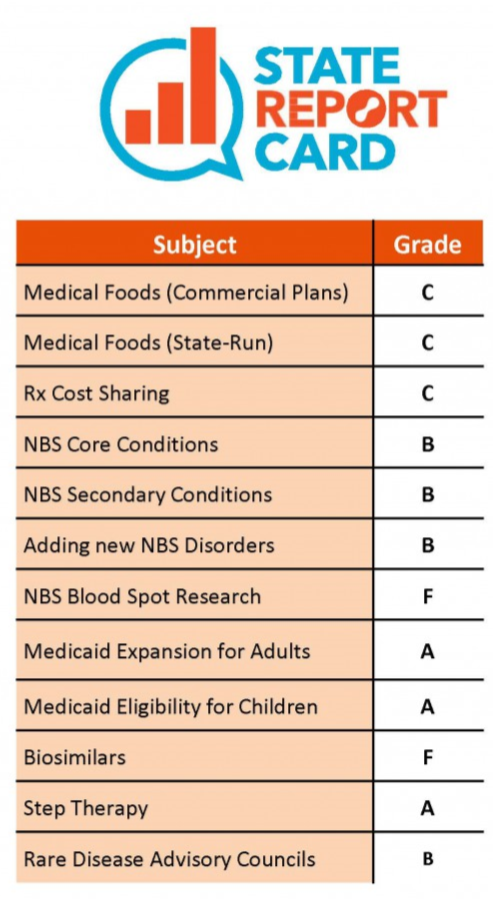
What is a rare disease? Any disease, disorder, illness or condition affecting fewer than 200,000 people in the United States is considered rare. It is estimated that 7,000 rare diseases exist, and fewer than 500 have FDA-approved treatments. Patients with rare diseases are frequently misdiagnosed or undiagnosed. Currently, only 5 percent of rare diseases have treatments, NORD points out.
A majority of states are not measuring up on legislative solutions that reduce the burden of rare diseases affecting 30 million Americans, according to a new report released by the the NORD Rare Action Network® (NORD RAN). The 2018 “State Report Card” indicates that progress in several areas of health policy is slow, according to the report.
The third annual edition of the State Report Card rates states on the strength of policies including coverage of medical foods and newborn screening, prescription drug cost-sharing limits, policies supporting biosimilar prescriber communications, protections against step therapy protocols, and the establishment of rare disease advisory councils. New this year, according to NORD, the report also looks at Medicaid Waivers (including proposed work requirements, lifetime limits, drug formulary restrictions, and other proposed changes to benefits), storage and research consent for dried blood spot samples used in newborn screening, and state Right-to-Try laws.
Connecticut earned grades all across the scale - three A's and four B's, as well as three C's and two F's. Overall, the report found nationally that:
- Fifteen states earned an F for failing to mandate adequate coverage of medical foods
- Thirty-six states earned an F for failing to enact prescription drug cost-sharing limits, despite third-party analysis showing these cause little to no impact on overall plan premiums for all beneficiaries
- Newborn screening has saved tens of thousands of lives, yet more than half of states fail to meet federal recommendations
- Fifteen states (including Connecticut) earned an A or B for protecting patients against step therapy, a procedure by which insurers (public or private) interfere with and delay appropriate care for patients that ultimately increases costs
“The intent with this report is to share valuable information that will enable advocates to affect change in their state,” said NORD Director of State Policy, Tim Boyd. “Our goal is to provide actionable steps for states that will improve people’s lives, so the report presents findings as well as tools for individuals to act on.”
Under the Affordable Care Act, many people with rare diseases can now access affordable health insurance. However, NORD officials point out, some insurance policies place orphan therapies on the so-called “specialty-tier” of a drug formulary. For drugs placed on this tier, enrollees often must meet cost sharing requirements that can be as much as 50% of the actual cost of the medication.

Later this month, NORD marks its 35th anniversary with a Summit in Washington, DC. A 501(c)(3) organization, NORD is a patient advocacy organization dedicated to individuals with rare diseases and the organizations that serve them. NORD, along with its more than 280 patient organization members, is committed to the identification, treatment, and cure of rare disorders through programs of education, advocacy, research, and patient services.